Dr Karen Lebacqz, Ph.D. On The Belmont Report – A Code Of BioMedical Ethics, Morals And Guidelines For The Protection Of Humans, Who Are Subjects Of BioMedical Research – Requires Full Informed Consent, Respect for Persons, Beneficence, Justice, Doing No Harm, Maximizing Benefits, Minimizing Risks
Dr Karen Lebacqz, Ph.D. On The Belmont Report - A Code Of BioMedical Ethics, Morals And Guidelines For The Protection Of Humans, Who Are Subjects Of BioMedical Vaccine/Drug Research - Requires Full Informed Consent, Respect for Persons, Beneficence, Justice, Doing No Harm, Maximizing Benefits, Minimizing Risks
WHAT IS FULL INFORMED CONSENT?
VIDEO: https://youtu.be/u5mRVQ3coIk 5 min
Today's MedCat video covers the Belmont Report (1979) that delineated the following three ethical tenets of human subjects research: respect for persons, beneficence, and justice. It is of medium-low yield for the MCAT, but highly important in medical research."
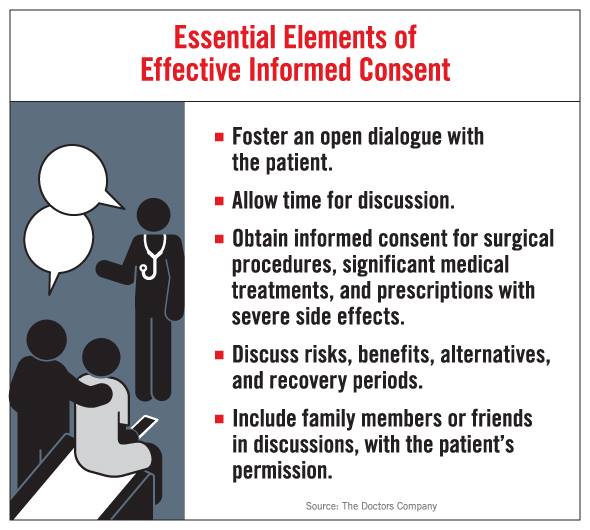
This article explores in depth the differences between 'manufactured' consent, coercion, the corrupting profit motive, and full willing, informed VOLUNTARY consent, through the 'lens' of the Belmont Report.
The Belmont Report is built on top of and includes the Hippocratic Oath, which mandates that a physician, nurse or other medical professional DO NO HARM.

What does it mean to 'do no harm'?
Go deeper
Physicians for Informed Consent (PIC) Mandatory Vaccination Laws Without Exemptions Violate Human and Civil Rights - What Is Full Informed Consent? Bill Gates, 10 Billion Dollars And Manufactured Consent By Suzanne Humphries MD - Vaccines Violate Numerous Natural Laws, And Constitute Waging War Against Nature
https://www.agreenroadjournal.com/2019/03/physicians-for-informed-consent-pic.html
FORCED VACCINATION VIOLATES BODILY AUTONOMY AND INFORMED CONSENT AND CAUSES 'HARM'
VIDEO: https://youtu.be/OSGxf_lJr_0 53 min
Forced vaccinations are like forced medical procedures, or they are like rape, in that they violate bodily integrity and full informed consent.
Go deeper
Assoc. of American Physicians/Surgeons (AAPS), Physicians for Informed Consent, Medical Nurses Are ALL Against Mandatory Vaccines, Violate Human Rights - Dr Mercola; Why Do People Endure 92 Percent Failure Rate With Flu Vaccines? Poland/Sweden Ban Mandatory Vaccinations - Mandatory Vaccines Are In Direct Violation Of Nuremberg Code
https://www.agreenroadjournal.com/2019/03/forced-vaccination-unnecessary-violates.html
TEDX - LACK OF ETHICS VIA INDUSTRY FUNDED RESEARCH AND REPORTS CAUSES HARM AND VIOLATIONS OF MEDICAL ETHICS 'RULES', LAWS, INTERNATIONAL AGREEMENTS SUCH AS NUREMBERG CODE
VIDEO: https://youtu.be/JSV4VZ8gdUQ 12 min
What if the researchers we trust to keep us safe are having their work influenced by hidden biases? Garry Gray is a University of Victoria Sociology professor and a Network Fellow at the Edmond J. Safra Center for Ethics at Harvard University."
What is the result of the PROFIT motive inside of scientific research?
What if certain questions are not answered or asked, due to pressure from funding sources?
What effect does industry funding have on scientists studying the industry focused product or service?
What happens when ethical lapses combine together and how do they affect trust in a wide range of technologies, food, drinks, medical products?
Go deeper
Dr.Sherri Tenpenny: All Emergency Use Authorization (EUA) Covid “Vaccines” Are Violating Multiple Laws, Illegal Poison Ingredients - OSHA Reversed Policy On Experimental Products Harm/Deaths, But Why? G Edward Griffin On The Enslavement Of Humanity In The Age Of Faucism - Red Pill Versus Blue Pill, Which Is Better? Dr. Byram Bridle On Fertility, Infants, Breastfeeding, Blood Donation
https://www.agreenroadjournal.com/2021/06/drsherri-tenpenny-emergency.html
WHAT IS THE BELMONT REPORT?
VIDEO: https://youtu.be/86zWBjDaXPk 5 min
The Belmont Report (Part Two: Applying the Principles)
VIDEO: https://youtu.be/cIafASIIU70 5 min
The Belmont Report
Office of the Secretary
Ethical Principles and Guidelines for the Protection of Human Subjects of Research
The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research
April 18, 1979
Printable PDF version
AGENCY: Department of Health, Education, and Welfare.
SUMMARY: On July 12, 1974, the National Research Act (Pub. L. 93-348) was signed into law, there-by creating the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. One of the charges to the Commission was to identify the basic ethical principles that should underlie the conduct of biomedical and behavioral research involving human subjects and to develop guidelines which should be followed to assure that such research is conducted in accordance with those principles. In carrying out the above, the Commission was directed to consider: (i) the boundaries between biomedical and behavioral research and the accepted and routine practice of medicine, (ii) the role of assessment of risk-benefit criteria in the determination of the appropriateness of research involving human subjects, (iii) appropriate guidelines for the selection of human subjects for participation in such research and (iv) the nature and definition of informed consent in various research settings.
The Belmont Report attempts to summarize the basic ethical principles identified by the Commission in the course of its deliberations. It is the outgrowth of an intensive four-day period of discussions that were held in February 1976 at the Smithsonian Institution's Belmont Conference Center supplemented by the monthly deliberations of the Commission that were held over a period of nearly four years. It is a statement of basic ethical principles and guidelines that should assist in resolving the ethical problems that surround the conduct of research with human subjects. By publishing the Report in the Federal Register, and providing reprints upon request, the Secretary intends that it may be made readily available to scientists, members of Institutional Review Boards, and Federal employees. The two-volume Appendix, containing the lengthy reports of experts and specialists who assisted the Commission in fulfilling this part of its charge, is available as DHEW Publication No. (OS) 78-0013 and No. (OS) 78-0014, for sale by the Superintendent of Documents, U.S. Government Printing Office, Washington, D.C. 20402.
Unlike most other reports of the Commission, the Belmont Report does not make specific recommendations for administrative action by the Secretary of Health, Education, and Welfare. Rather, the Commission recommended that the Belmont Report be adopted in its entirety, as a statement of the Department's policy. The Department requests public comment on this recommendation.
National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research
MEMBERS OF COMMISSION
Members of the Commission
Kenneth John Ryan, M.D., Chairman, Chief of Staff, Boston Hospital for Women.
Joseph V. Brady, Ph.D., Professor of Behavioral Biology, Johns Hopkins University.
Robert E. Cooke, M.D., President, Medical College of Pennsylvania.
Dorothy I. Height, President, National Council of Negro Women, Inc.
Albert R. Jonsen, Ph.D., Associate Professor of Bioethics, University of California at San Francisco.
Patricia King, J.D., Associate Professor of Law, Georgetown University Law Center.
Karen Lebacqz, Ph.D., Associate Professor of Christian Ethics, Pacific School of Religion.
*** David W. Louisell, J.D., Professor of Law, University of California at Berkeley.
Donald W. Seldin, M.D., Professor and Chairman, Department of Internal Medicine, University of Texas at Dallas.
***Eliot Stellar, Ph.D., Provost of the University and Professor of Physiological Psychology, University of Pennsylvania.
*** Robert H. Turtle, LL.B., Attorney, VomBaur, Coburn, Simmons & Turtle, Washington, D.C.*** Deceased.
ETHICAL PRINCIPLES AND GUIDELINES FOR RESEARCH INVOLVING HUMAN SUBJECTS
Scientific research has produced substantial social benefits. It has also posed some troubling ethical questions. Public attention was drawn to these questions by reported abuses of human subjects in biomedical experiments, especially during the Second World War. During the Nuremberg War Crime Trials, the Nuremberg code was drafted as a set of standards for judging physicians and scientists who had conducted biomedical experiments on concentration camp prisoners. This code became the prototype of many later codes[1] intended to assure that research involving human subjects would be carried out in an ethical manner.
The codes consist of rules, some general, others specific, that guide the investigators or the reviewers of research in their work. Such rules often are inadequate to cover complex situations; at times they come into conflict, and they are frequently difficult to interpret or apply. Broader ethical principles will provide a basis on which specific rules may be formulated, criticized and interpreted.
Three principles, or general prescriptive judgments, that are relevant to research involving human subjects are identified in this statement. Other principles may also be relevant. These three are comprehensive, however, and are stated at a level of generalization that should assist scientists, subjects, reviewers and interested citizens to understand the ethical issues inherent in research involving human subjects. These principles cannot always be applied so as to resolve beyond dispute particular ethical problems. The objective is to provide an analytical framework that will guide the resolution of ethical problems arising from research involving human subjects.
This statement consists of a distinction between research and practice, a discussion of the three basic ethical principles, and remarks about the application of these principles.
BOUNDARIES BETWEEN PRACTICE AND RESEARCH
A. Boundaries Between Practice and Research
It is important to distinguish between biomedical and behavioral research, on the one hand, and the practice of accepted therapy on the other, in order to know what activities ought to undergo review for the protection of human subjects of research. The distinction between research and practice is blurred partly because both often occur together (as in research designed to evaluate a therapy) and partly because notable departures from standard practice are often called "experimental" when the terms "experimental" and "research" are not carefully defined.
For the most part, the term "practice" refers to interventions that are designed solely to enhance the well-being of an individual patient or client and that have a reasonable expectation of success. The purpose of medical or behavioral practice is to provide diagnosis, preventive treatment or therapy to particular individuals [2]. By contrast, the term "research' designates an activity designed to test an hypothesis, permit conclusions to be drawn, and thereby to develop or contribute to generalizable knowledge (expressed, for example, in theories, principles, and statements of relationships). Research is usually described in a formal protocol that sets forth an objective and a set of procedures designed to reach that objective.
When a clinician departs in a significant way from standard or accepted practice, the innovation does not, in and of itself, constitute research. The fact that a procedure is "experimental," in the sense of new, untested or different, does not automatically place it in the category of research. Radically new procedures of this description should, however, be made the object of formal research at an early stage in order to determine whether they are safe and effective. Thus, it is the responsibility of medical practice committees, for example, to insist that a major innovation be incorporated into a formal research project [3].
Research and practice may be carried on together when research is designed to evaluate the safety and efficacy of a therapy. This need not cause any confusion regarding whether or not the activity requires review; the general rule is that if there is any element of research in an activity, that activity should undergo review for the protection of human subjects.
BASIC ETHICAL PRINCIPLES
B. Basic Ethical Principles
The expression "basic ethical principles" refers to those general judgments that serve as a basic justification for the many particular ethical prescriptions and evaluations of human actions. Three basic principles, among those generally accepted in our cultural tradition, are particularly relevant to the ethics of research involving human subjects: the principles of respect of persons, beneficence and justice.
1. Respect for Persons. -- Respect for persons incorporates at least two ethical convictions: first, that individuals should be treated as autonomous agents, and second, that persons with diminished autonomy are entitled to protection. The principle of respect for persons thus divides into two separate moral requirements: the requirement to acknowledge autonomy and the requirement to protect those with diminished autonomy.
An autonomous person is an individual capable of deliberation about personal goals and of acting under the direction of such deliberation. To respect autonomy is to give weight to autonomous persons' considered opinions and choices while refraining from obstructing their actions unless they are clearly detrimental to others. To show lack of respect for an autonomous agent is to repudiate that person's considered judgments, to deny an individual the freedom to act on those considered judgments, or to withhold information necessary to make a considered judgment, when there are no compelling reasons to do so.
However, not every human being is capable of self-determination. The capacity for self-determination matures during an individual's life, and some individuals lose this capacity wholly or in part because of illness, mental disability, or circumstances that severely restrict liberty. Respect for the immature and the incapacitated may require protecting them as they mature or while they are incapacitated.
Some persons are in need of extensive protection, even to the point of excluding them from activities which may harm them; other persons require little protection beyond making sure they undertake activities freely and with awareness of possible adverse consequence. The extent of protection afforded should depend upon the risk of harm and the likelihood of benefit. The judgment that any individual lacks autonomy should be periodically reevaluated and will vary in different situations.
In most cases of research involving human subjects, respect for persons demands that subjects enter into the research voluntarily and with adequate information. In some situations, however, application of the principle is not obvious. The involvement of prisoners as subjects of research provides an instructive example. On the one hand, it would seem that the principle of respect for persons requires that prisoners not be deprived of the opportunity to volunteer for research. On the other hand, under prison conditions they may be subtly coerced or unduly influenced to engage in research activities for which they would not otherwise volunteer. Respect for persons would then dictate that prisoners be protected. Whether to allow prisoners to "volunteer" or to "protect" them presents a dilemma. Respecting persons, in most hard cases, is often a matter of balancing competing claims urged by the principle of respect itself.
2. Beneficence. -- Persons are treated in an ethical manner not only by respecting their decisions and protecting them from harm, but also by making efforts to secure their well-being. Such treatment falls under the principle of beneficence. The term "beneficence" is often understood to cover acts of kindness or charity that go beyond strict obligation. In this document, beneficence is understood in a stronger sense, as an obligation. Two general rules have been formulated as complementary expressions of beneficent actions in this sense: (1) do not harm and (2) maximize possible benefits and minimize possible harms.
The Hippocratic maxim "do no harm" has long been a fundamental principle of medical ethics. Claude Bernard extended it to the realm of research, saying that one should not injure one person regardless of the benefits that might come to others. However, even avoiding harm requires learning what is harmful; and, in the process of obtaining this information, persons may be exposed to risk of harm. Further, the Hippocratic Oath requires physicians to benefit their patients "according to their best judgment." Learning what will in fact benefit may require exposing persons to risk. The problem posed by these imperatives is to decide when it is justifiable to seek certain benefits despite the risks involved, and when the benefits should be foregone because of the risks.
The obligations of beneficence affect both individual investigators and society at large, because they extend both to particular research projects and to the entire enterprise of research. In the case of particular projects, investigators and members of their institutions are obliged to give forethought to the maximization of benefits and the reduction of risk that might occur from the research investigation. In the case of scientific research in general, members of the larger society are obliged to recognize the longer term benefits and risks that may result from the improvement of knowledge and from the development of novel medical, psychotherapeutic, and social procedures.
The principle of beneficence often occupies a well-defined justifying role in many areas of research involving human subjects. An example is found in research involving children. Effective ways of treating childhood diseases and fostering healthy development are benefits that serve to justify research involving children -- even when individual research subjects are not direct beneficiaries. Research also makes it possible to avoid the harm that may result from the application of previously accepted routine practices that on closer investigation turn out to be dangerous. But the role of the principle of beneficence is not always so unambiguous. A difficult ethical problem remains, for example, about research that presents more than minimal risk without immediate prospect of direct benefit to the children involved. Some have argued that such research is inadmissible, while others have pointed out that this limit would rule out much research promising great benefit to children in the future. Here again, as with all hard cases, the different claims covered by the principle of beneficence may come into conflict and force difficult choices.
3. Justice. -- Who ought to receive the benefits of research and bear its burdens? This is a question of justice, in the sense of "fairness in distribution" or "what is deserved." An injustice occurs when some benefit to which a person is entitled is denied without good reason or when some burden is imposed unduly. Another way of conceiving the principle of justice is that equals ought to be treated equally. However, this statement requires explication. Who is equal and who is unequal? What considerations justify departure from equal distribution? Almost all commentators allow that distinctions based on experience, age, deprivation, competence, merit and position do sometimes constitute criteria justifying differential treatment for certain purposes. It is necessary, then, to explain in what respects people should be treated equally. There are several widely accepted formulations of just ways to distribute burdens and benefits. Each formulation mentions some relevant property on the basis of which burdens and benefits should be distributed. These formulations are (1) to each person an equal share, (2) to each person according to individual need, (3) to each person according to individual effort, (4) to each person according to societal contribution, and (5) to each person according to merit.
Questions of justice have long been associated with social practices such as punishment, taxation and political representation. Until recently these questions have not generally been associated with scientific research. However, they are foreshadowed even in the earliest reflections on the ethics of research involving human subjects. For example, during the 19th and early 20th centuries the burdens of serving as research subjects fell largely upon poor ward patients, while the benefits of improved medical care flowed primarily to private patients. Subsequently, the exploitation of unwilling prisoners as research subjects in Nazi concentration camps was condemned as a particularly flagrant injustice. In this country, in the 1940's, the Tuskegee syphilis study used disadvantaged, rural black men to study the untreated course of a disease that is by no means confined to that population. These subjects were deprived of demonstrably effective treatment in order not to interrupt the project, long after such treatment became generally available.
Against this historical background, it can be seen how conceptions of justice are relevant to research involving human subjects. For example, the selection of research subjects needs to be scrutinized in order to determine whether some classes (e.g., welfare patients, particular racial and ethnic minorities, or persons confined to institutions) are being systematically selected simply because of their easy availability, their compromised position, or their manipulability, rather than for reasons directly related to the problem being studied. Finally, whenever research supported by public funds leads to the development of therapeutic devices and procedures, justice demands both that these not provide advantages only to those who can afford them and that such research should not unduly involve persons from groups unlikely to be among the beneficiaries of subsequent applications of the research.
APPLICATIONS
C. Applications
Applications of the general principles to the conduct of research leads to consideration of the following requirements: informed consent, risk/benefit assessment, and the selection of subjects of research.
1. Informed Consent. -- Respect for persons requires that subjects, to the degree that they are capable, be given the opportunity to choose what shall or shall not happen to them. This opportunity is provided when adequate standards for informed consent are satisfied.
While the importance of informed consent is unquestioned, controversy prevails over the nature and possibility of an informed consent. Nonetheless, there is widespread agreement that the consent process can be analyzed as containing three elements: information, comprehension and voluntariness.
Information. Most codes of research establish specific items for disclosure intended to assure that subjects are given sufficient information. These items generally include: the research procedure, their purposes, risks and anticipated benefits, alternative procedures (where therapy is involved), and a statement offering the subject the opportunity to ask questions and to withdraw at any time from the research. Additional items have been proposed, including how subjects are selected, the person responsible for the research, etc.
However, a simple listing of items does not answer the question of what the standard should be for judging how much and what sort of information should be provided. One standard frequently invoked in medical practice, namely the information commonly provided by practitioners in the field or in the locale, is inadequate since research takes place precisely when a common understanding does not exist. Another standard, currently popular in malpractice law, requires the practitioner to reveal the information that reasonable persons would wish to know in order to make a decision regarding their care. This, too, seems insufficient since the research subject, being in essence a volunteer, may wish to know considerably more about risks gratuitously undertaken than do patients who deliver themselves into the hand of a clinician for needed care. It may be that a standard of "the reasonable volunteer" should be proposed: the extent and nature of information should be such that persons, knowing that the procedure is neither necessary for their care nor perhaps fully understood, can decide whether they wish to participate in the furthering of knowledge. Even when some direct benefit to them is anticipated, the subjects should understand clearly the range of risk and the voluntary nature of participation.
A special problem of consent arises where informing subjects of some pertinent aspect of the research is likely to impair the validity of the research. In many cases, it is sufficient to indicate to subjects that they are being invited to participate in research of which some features will not be revealed until the research is concluded. In all cases of research involving incomplete disclosure, such research is justified only if it is clear that (1) incomplete disclosure is truly necessary to accomplish the goals of the research, (2) there are no undisclosed risks to subjects that are more than minimal, and (3) there is an adequate plan for debriefing subjects, when appropriate, and for dissemination of research results to them. Information about risks should never be withheld for the purpose of eliciting the cooperation of subjects, and truthful answers should always be given to direct questions about the research. Care should be taken to distinguish cases in which disclosure would destroy or invalidate the research from cases in which disclosure would simply inconvenience the investigator.
Comprehension. The manner and context in which information is conveyed is as important as the information itself. For example, presenting information in a disorganized and rapid fashion, allowing too little time for consideration or curtailing opportunities for questioning, all may adversely affect a subject's ability to make an informed choice.
Because the subject's ability to understand is a function of intelligence, rationality, maturity and language, it is necessary to adapt the presentation of the information to the subject's capacities. Investigators are responsible for ascertaining that the subject has comprehended the information. While there is always an obligation to ascertain that the information about risk to subjects is complete and adequately comprehended, when the risks are more serious, that obligation increases. On occasion, it may be suitable to give some oral or written tests of comprehension.
Special provision may need to be made when comprehension is severely limited -- for example, by conditions of immaturity or mental disability. Each class of subjects that one might consider as incompetent (e.g., infants and young children, mentally disable patients, the terminally ill and the comatose) should be considered on its own terms. Even for these persons, however, respect requires giving them the opportunity to choose to the extent they are able, whether or not to participate in research. The objections of these subjects to involvement should be honored, unless the research entails providing them a therapy unavailable elsewhere. Respect for persons also requires seeking the permission of other parties in order to protect the subjects from harm. Such persons are thus respected both by acknowledging their own wishes and by the use of third parties to protect them from harm. (PARENTS OF CHILDREN)
The third parties chosen should be those who are most likely to understand the incompetent subject's situation and to act in that person's best interest. The person authorized to act on behalf of the subject should be given an opportunity to observe the research as it proceeds in order to be able to withdraw the subject from the research, if such action appears in the subject's best interest.
Voluntariness. An agreement to participate in research constitutes a valid consent only if voluntarily given. This element of informed consent requires conditions free of coercion and undue influence. Coercion occurs when an overt threat of harm is intentionally presented by one person to another in order to obtain compliance. Undue influence, by contrast, occurs through an offer of an excessive, unwarranted, inappropriate or improper reward or other overture in order to obtain compliance. Also, inducements that would ordinarily be acceptable may become undue influences if the subject is especially vulnerable.
Unjustifiable pressures usually occur when persons in positions of authority or commanding influence -- especially where possible sanctions are involved -- urge a course of action for a subject. A continuum of such influencing factors exists, however, and it is impossible to state precisely where justifiable persuasion ends and undue influence begins. But undue influence would include actions such as manipulating a person's choice through the controlling influence of a close relative and threatening to withdraw health services to which an individual would otherwise be entitled.
2. Assessment of Risks and Benefits. -- The assessment of risks and benefits requires a careful arrayal of relevant data, including, in some cases, alternative ways of obtaining the benefits sought in the research. Thus, the assessment presents both an opportunity and a responsibility to gather systematic and comprehensive information about proposed research. For the investigator, it is a means to examine whether the proposed research is properly designed. For a review committee, it is a method for determining whether the risks that will be presented to subjects are justified. For prospective subjects, the assessment will assist the determination whether or not to participate.
The Nature and Scope of Risks and Benefits. The requirement that research be justified on the basis of a favorable risk/benefit assessment bears a close relation to the principle of beneficence, just as the moral requirement that informed consent be obtained is derived primarily from the principle of respect for persons. The term "risk" refers to a possibility that harm may occur. However, when expressions such as "small risk" or "high risk" are used, they usually refer (often ambiguously) both to the chance (probability) of experiencing a harm and the severity (magnitude) of the envisioned harm.
The term "benefit" is used in the research context to refer to something of positive value related to health or welfare. Unlike, "risk," "benefit" is not a term that expresses probabilities. Risk is properly contrasted to probability of benefits, and benefits are properly contrasted with harms rather than risks of harm. Accordingly, so-called risk/benefit assessments are concerned with the probabilities and magnitudes of possible harm and anticipated benefits. Many kinds of possible harms and benefits need to be taken into account. There are, for example, risks of psychological harm, physical harm, legal harm, social harm and economic harm and the corresponding benefits. While the most likely types of harms to research subjects are those of psychological or physical pain or injury, other possible kinds should not be overlooked.
Risks and benefits of research may affect the individual subjects, the families of the individual subjects, and society at large (or special groups of subjects in society). Previous codes and Federal regulations have required that risks to subjects be outweighed by the sum of both the anticipated benefit to the subject, if any, and the anticipated benefit to society in the form of knowledge to be gained from the research. In balancing these different elements, the risks and benefits affecting the immediate research subject will normally carry special weight. On the other hand, interests other than those of the subject may on some occasions be sufficient by themselves to justify the risks involved in the research, so long as the subjects' rights have been protected. Beneficence thus requires that we protect against risk of harm to subjects and also that we be concerned about the loss of the substantial benefits that might be gained from research.
The Systematic Assessment of Risks and Benefits. It is commonly said that benefits and risks must be "balanced" and shown to be "in a favorable ratio." The metaphorical character of these terms draws attention to the difficulty of making precise judgments. Only on rare occasions will quantitative techniques be available for the scrutiny of research protocols. However, the idea of systematic, nonarbitrary analysis of risks and benefits should be emulated insofar as possible. This ideal requires those making decisions about the justifiability of research to be thorough in the accumulation and assessment of information about all aspects of the research, and to consider alternatives systematically. This procedure renders the assessment of research more rigorous and precise, while making communication between review board members and investigators less subject to misinterpretation, misinformation and conflicting judgments. Thus, there should first be a determination of the validity of the presuppositions of the research; then the nature, probability and magnitude of risk should be distinguished with as much clarity as possible. The method of ascertaining risks should be explicit, especially where there is no alternative to the use of such vague categories as small or slight risk. It should also be determined whether an investigator's estimates of the probability of harm or benefits are reasonable, as judged by known facts or other available studies.
Finally, assessment of the justifiability of research should reflect at least the following considerations: (i) Brutal or inhumane treatment of human subjects is never morally justified. (ii) Risks should be reduced to those necessary to achieve the research objective. It should be determined whether it is in fact necessary to use human subjects at all. Risk can perhaps never be entirely eliminated, but it can often be reduced by careful attention to alternative procedures. (iii) When research involves significant risk of serious impairment, review committees should be extraordinarily insistent on the justification of the risk (looking usually to the likelihood of benefit to the subject -- or, in some rare cases, to the manifest voluntariness of the participation). (iv) When vulnerable populations are involved in research, the appropriateness of involving them should itself be demonstrated. A number of variables go into such judgments, including the nature and degree of risk, the condition of the particular population involved, and the nature and level of the anticipated benefits. (v) Relevant risks and benefits must be thoroughly arrayed in documents and procedures used in the informed consent process.
3. Selection of Subjects. -- Just as the principle of respect for persons finds expression in the requirements for consent, and the principle of beneficence in risk/benefit assessment, the principle of justice gives rise to moral requirements that there be fair procedures and outcomes in the selection of research subjects.
Justice is relevant to the selection of subjects of research at two levels: the social and the individual. Individual justice in the selection of subjects would require that researchers exhibit fairness: thus, they should not offer potentially beneficial research only to some patients who are in their favor or select only "undesirable" persons for risky research. Social justice requires that distinction be drawn between classes of subjects that ought, and ought not, to participate in any particular kind of research, based on the ability of members of that class to bear burdens and on the appropriateness of placing further burdens on already burdened persons. Thus, it can be considered a matter of social justice that there is an order of preference in the selection of classes of subjects (e.g., adults before children) and that some classes of potential subjects (e.g., the institutionalized mentally infirm or prisoners) may be involved as research subjects, if at all, only on certain conditions.
Injustice may appear in the selection of subjects, even if individual subjects are selected fairly by investigators and treated fairly in the course of research. Thus injustice arises from social, racial, sexual and cultural biases institutionalized in society. Thus, even if individual researchers are treating their research subjects fairly, and even if IRBs are taking care to assure that subjects are selected fairly within a particular institution, unjust social patterns may nevertheless appear in the overall distribution of the burdens and benefits of research. Although individual institutions or investigators may not be able to resolve a problem that is pervasive in their social setting, they can consider distributive justice in selecting research subjects.
Some populations, especially institutionalized ones, are already burdened in many ways by their infirmities and environments. When research is proposed that involves risks and does not include a therapeutic component, other less burdened classes of persons should be called upon first to accept these risks of research, except where the research is directly related to the specific conditions of the class involved. Also, even though public funds for research may often flow in the same directions as public funds for health care, it seems unfair that populations dependent on public health care constitute a pool of preferred research subjects if more advantaged populations are likely to be the recipients of the benefits.
One special instance of injustice results from the involvement of vulnerable subjects. Certain groups, such as racial minorities, the economically disadvantaged, the very sick, and the institutionalized may continually be sought as research subjects, owing to their ready availability in settings where research is conducted. Given their dependent status and their frequently compromised capacity for free consent, they should be protected against the danger of being involved in research solely for administrative convenience, or because they are easy to manipulate as a result of their illness or socioeconomic condition.
BELMONT REPORT INCLUDES AND IS EMBEDDED INSIDE OF NUREMBERG CODE, HELSINKI DECLARATION, US DEPT OF HEALTH GUIDELINES
[1] Since 1945, various codes for the proper and responsible conduct of human experimentation in medical research have been adopted by different organizations. The best known of these codes are the Nuremberg Code of 1947, the Helsinki Declaration of 1964 (revised in 1975), and the 1971 Guidelines (codified into Federal Regulations in 1974) issued by the U.S. Department of Health, Education, and Welfare Codes for the conduct of social and behavioral research have also been adopted, the best known being that of the American Psychological Association, published in 1973.
[2] Although practice usually involves interventions designed solely to enhance the well-being of a particular individual, interventions are sometimes applied to one individual for the enhancement of the well-being of another (e.g., blood donation, skin grafts, organ transplants) or an intervention may have the dual purpose of enhancing the well-being of a particular individual, and, at the same time, providing some benefit to others (e.g., vaccination, which protects both the person who is vaccinated and society generally). The fact that some forms of practice have elements other than immediate benefit to the individual receiving an intervention, however, should not confuse the general distinction between research and practice. Even when a procedure applied in practice may benefit some other person, it remains an intervention designed to enhance the well-being of a particular individual or groups of individuals; thus, it is practice and need not be reviewed as research.
[3] Because the problems related to social experimentation may differ substantially from those of biomedical and behavioral research, the Commission specifically declines to make any policy determination regarding such research at this time. Rather, the Commission believes that the problem ought to be addressed by one of its successor bodies.
https://www.hhs.gov/ohrp/regulations-and-policy/belmont-report/read-the-belmont-report/index.html
Go deeper
Victor Restrepo: Natural Law Based Precautionary Principle vs Short Term Greed/Profit Approach - What Works For 7 Future Generations Without Causing Harm? The Pressure Is Growing To Adopt The Principle Of Debating Effect On 7 Future Generations, Or Suffer Severe Consequences
https://www.agreenroadjournal.com/2015/04/victor-restrepo-precautionary-principle.html
WHAT IS LACK OF INFORMED CONSENT? COERCION VIA PRESSURE, GIFTS, THREATS, INTIMIDATION, CONSTANT MASS MEDIA MANIPULATION USING FEARMONGERING, PROPAGANDA AND MISINFORMATION DESIGNED TO IMPROVE 'PROFITS' FOR BILLIONAIRES
Thanks to: https://www.agreenroadjournal.com






 Sat Mar 23, 2024 11:33 pm by globalturbo
Sat Mar 23, 2024 11:33 pm by globalturbo

